Our Science
Pioneering a new kind of genomic medicine through in vivo hematopoietic stem cell (HSC) engineering

Our focus is one-time, off-the-shelf therapies that redesign the hematopoietic system to treat diseases with unprecedented versatility. We achieve this by directly engineering the master cells of this system – HSCs – in vivo to create a durable supply of therapeutic cells throughout the body and treat the cause of disease.
HSCs
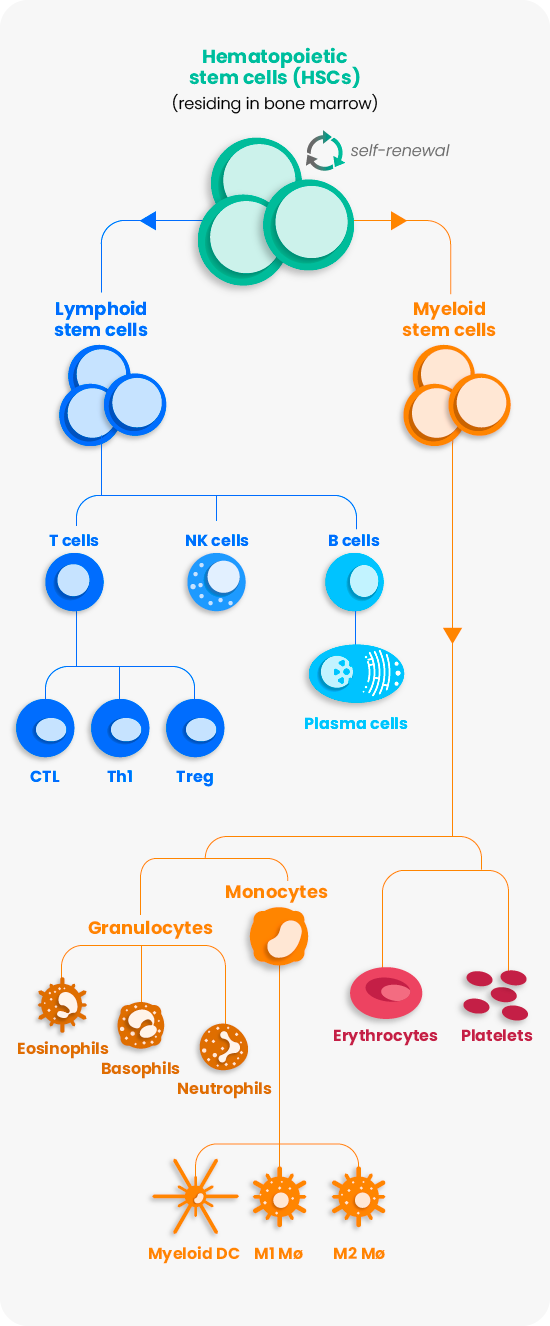
Harnessing the master cell
HSCs continuously self-renew throughout our lives and differentiate into many specialized blood and immune cells which make up the hematopoietic system. This natural versatility has led to their use in the treatment of hundreds of diseases. As the leader in engineering HSCs in vivo, we leverage the natural function of the entire hematopoietic system.
With our platform, we aim to supersede cell therapy approaches focused on single cell types that are transiently present by controlling a diverse cellular response against disease.


Exceptional delivery, sophisticated engineering
By contrast to ex vivo approaches that engineer cells outside the body through a complex manufacturing process before reintroducing them into patients, our platform delivers the engineering tools directly to cells within the body.
To achieve this, we combine an in vivo off-the-shelf delivery system using virus-like particles (VLPs) with an advanced gene engineering toolkit.
Delivery: Our Virus-Like Particles
- Target HSCs, not the liver, for lower dosing and safety
- Have viral genes removed for added safety and capacity
- Deliver several times more genetic material than other approaches, enabling many more therapeutic designs
- Deliver genetic material to the nucleus, not just the cytoplasm
- Are reproducibly manufactured to GMP with scalability to thousands of liters
Engineering: Our In Vivo Toolkit
- Enables a full range of precise genetic changes, from single nucleotide to large gene insertions
- Includes proprietary CRISPR-Cas platform and class-leading base editors that do not cut DNA
- Synergizes with VLP capacity to enable multiple gene insertions and edits at once for multifunctional engineered cells
- Enables lineage-specific gene expression in desired HSC progenitor cells
- Supports self-renewing and durable engineered HSCs
Robust & scalable manufacturing process
The proven scalability of our VLP production opens the possibility of serving large patient populations globally through production centers in many locations. We have demonstrated consistency across multiple clinical-scale batches and advanced analytics have shown our VLPs to be reliably potent and effective at delivering payloads to the nucleus of HSCs.
Looking to the future, our platform approach is designed to allow us to exchange genetic payloads within VLPs with ease for new indications while maintaining scale and productivity.

A treatment process that puts patients first
The patient experience is paramount. We aim to empower the cells in patients’ bodies through a process that could ultimately be entirely outpatient. Our goal is to remove the complex manufacturing and burdensome pre- and post-treatment processes of prior approaches. Through in vivo, off-the-shelf therapies, we aim to create genetic medicine that can reach more patients around the world than ever before.
Therapeutic strategies for our in vivo genetic medicines
We’re bringing the full power of the hematopoietic system to bear on challenging diseases. From small edits that make all the difference in genetic disease to multi-gene, multi-cellular designs to tackle cancer, we’re up for the challenge.

Gene insertion: X-linked chronic granulomatous disease (X-CGD)

X-CGD is caused by a mutant CYBB gene that prevents white blood cells called neutrophils from producing functional NADPH oxidase, which is important for fighting infection.
Our solution is to insert corrected CYBB into HSCs, with a genetic promoter that ensures the gene is expressed in neutrophils. Successful expression will restore the ability to fight infection. This is the first clinical in vivo HSC approach for X-CGD and leverages a mechanism validated ex vivo. It is designed to function for any CYBB mutation. In addition to therapeutic benefit, this program will validate the safety and efficacy of engineering HSCs; their manufacturing; and provide a modular gene insertion framework for further programs.
Multiplexed cell therapy: Solid tumors

Solid tumors have many strategies for evading or weakening the immune system, suggesting a need for a multi-cellular and long-lasting approach to overcome their defenses.
We are pursuing a multiplexed, multi-cell approach to this major area of medicine. Our therapeutic designs will coordinate a synergistic anti-tumor attack from several cell types, each expressing engineered proteins. This builds on the exciting promise seen with single-cell approaches such as engineered T cells, natural killer cells and macrophages. Our goal is to deliver a new benchmark for durable treatment outcomes in solid tumors.
Gene editing: Sickle cell disease (SCD)

SCD is caused by a hemoglobin mutation that creates malformation of red blood cells (RBCs), which causes a wide range of symptoms including anemia, pain crises and an increased risk of serious infections, stroke, and death.
Our solution is to precisely base edit HSCs and reactivate the fetal form of hemoglobin (HbF), which restores normal RBC form and function. This approach has been validated by an inherited condition where HbF persists as well as by ex vivo gene therapies. This de-risks our strategy to enhance the patient experience and outcomes by translating a known mechanism in vivo.